The Journal of Chemical Physics, AIP Publishing, 2023. 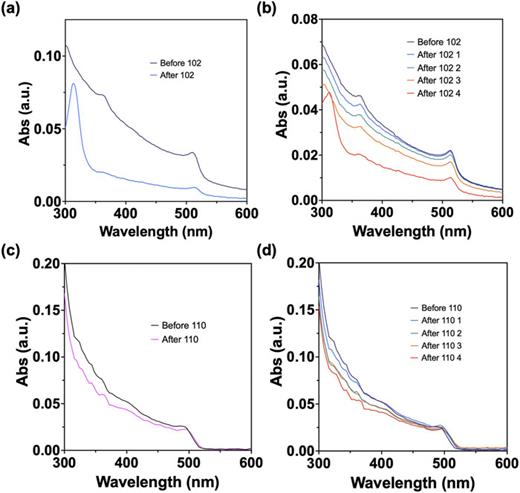
![link The surface chemistry of ionic liquid-treated CsPbBr3 quantum dots [link]](https://bibbase.org/img/filetypes/link.svg) Paper doi abstract bibtex 1 download
Paper doi abstract bibtex 1 download

![link The surface chemistry of ionic liquid-treated CsPbBr3 quantum dots [link]](https://bibbase.org/img/filetypes/link.svg) Paper doi abstract bibtex 1 download
Paper doi abstract bibtex 1 download The power conversion efficiencies of lead halide perovskite thin film solar cells have surged in the short time since their inception. Compounds, such as ionic liquids (ILs), have been explored as chemical additives and interface modifiers in perovskite solar cells, contributing to the rapid increase in cell efficiencies. However, due to the small surface area-to-volume ratio of the large grained polycrystalline halide perovskite films, an atomistic understanding of the interaction between ILs and perovskite surfaces is limited. Here, we use quantum dots (QDs) to study the coordinative surface interaction between phosphonium-based ILs and CsPbBr3. When native oleylammonium oleate ligands are exchanged off the QD surface with the phosphonium cation as well as the IL anion, a threefold increase in photoluminescent quantum yield of as-synthesized QDs is observed. The CsPbBr3 QD structure, shape, and size remain unchanged after ligand exchange, indicating only a surface ligand interaction at approximately equimolar additions of the IL. Increased concentrations of the IL lead to a disadvantageous phase change and a concomitant decrease in photoluminescent quantum yields. Valuable information regarding the coordinative interaction between certain ILs and lead halide perovskites has been elucidated and can be used for informed pairing of beneficial combinations of IL cations and anions.
@article{Crans_2023,
title={The surface chemistry of ionic liquid-treated CsPbBr3 quantum dots},
volume={158},
ISSN={1089-7690},
url={http://dx.doi.org/10.1063/5.0147918},
DOI={10.1063/5.0147918},
number={17},
journal={The Journal of Chemical Physics},
publisher={AIP Publishing},
author={Crans, Kyle D. and Bain, Matthew and Bradforth, Stephen E. and Oron, Dan and Kazes, Miri and Brutchey, Richard L.},
year={2023},
abstract = {The power conversion efficiencies of lead halide perovskite thin film solar cells have surged in the short time since their inception. Compounds, such as ionic liquids (ILs), have been explored as chemical additives and interface modifiers in perovskite solar cells, contributing to the rapid increase in cell efficiencies. However, due to the small surface area-to-volume ratio of the large grained polycrystalline halide perovskite films, an atomistic understanding of the interaction between ILs and perovskite surfaces is limited. Here, we use quantum dots (QDs) to study the coordinative surface interaction between phosphonium-based ILs and CsPbBr3. When native oleylammonium oleate ligands are exchanged off the QD surface with the phosphonium cation as well as the IL anion, a threefold increase in photoluminescent quantum yield of as-synthesized QDs is observed. The CsPbBr3 QD structure, shape, and size remain unchanged after ligand exchange, indicating only a surface ligand interaction at approximately equimolar additions of the IL. Increased concentrations of the IL lead to a disadvantageous phase change and a concomitant decrease in photoluminescent quantum yields. Valuable information regarding the coordinative interaction between certain ILs and lead halide perovskites has been elucidated and can be used for informed pairing of beneficial combinations of IL cations and anions.},
bibbase_note = {<img src="https://aipp.silverchair-cdn.com/aipp/content_public/journal/jcp/158/17/10.1063_5.0147918/1/m_174709_1_5.0147918.figures.online.f4.jpeg?Expires=1715519872&Signature=jFY81bFmvCr9RqEfC2AyUonsIjK1AF2yfEadkxGZfFHOpAYHLXWU9xvjfPlpF8iiyhdNKyMUzhXg~B0P5NcTk4VFkrFWdzWSxkznrHg5u1KQfGTM0hGkmRT7zKbSkBWAS1Rweov4N3lzfLdfcXAxIkoUX0aLZdSmtvsqjciQ7D2BqIiurB6NbXTg9I3PF~-NCXiBhmhT~XCGgjP6EIizg1ik8nUwsgZqu0MI68eEw1volArz4Rf1PJrtWF9MgCE7HUumrWca010TGjClovmek-ZXzWpfARCebHQZuG-hQTXg9gnIqJ44IfFYVRpTpONSJ4mHBksP-rsneBkumjG1BQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA">}
}Downloads: 1