Faraday Discussions, 216:379–394, Royal Society of Chemistry (RSC), 2019. 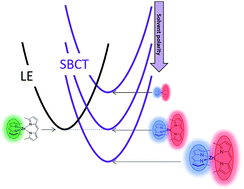
![link Symmetry breaking charge transfer as a means to study electron transfer with no driving force [link]](https://bibbase.org/img/filetypes/link.svg) Paper doi abstract bibtex
Paper doi abstract bibtex

![link Symmetry breaking charge transfer as a means to study electron transfer with no driving force [link]](https://bibbase.org/img/filetypes/link.svg) Paper doi abstract bibtex
Paper doi abstract bibtex Symmetry breaking charge transfer (SBCT) is a process where a symmetrically disposed pair of identical chromophores forms a charge transfer excited state with the hole and electron on different chromophores, i.e. chr–chr + hν → chr+–chr−. Herein we explore this process in two dipyrrin-based bichromophoric systems. One of these bisdipyrrins involved a pair of BODIPY chromophores linked by a single bond at their meso-positions (compound 1) and the other involved two dipyrrin ligands coordinated in a tetrahedral geometry at the Zn2+ ion (compound 2). Both compounds show rapid SBCT in polar solvents and only dipyrrin based emission in nonpolar solvents, the latter arising from a dipyrrin localized excited sate (LE). By “tuning” the solvent polarity the equilibrium between the LE and SBCT states can be shifted to favor either state. Ultrafast transient absorption spectroscopy (TA) was used to probe the kinetics of the charge transfer for 2 in solvents where the electron transfer is endergonic, exergonic and has a ΔG close to zero. Our TA derived rates were used to predict fluorescence efficiencies in each of the different solvent systems and showed a good correspondence to measured values. Detailed density functional theory (DFT) and time dependent DFT were used to model the ground states as well as the LE and SBCT states of 1 and 2, in both polar and nonpolar media. The ground and LE excited states show small dipole moments, while the SBCT states show dipole moments of 16.4 and 20.3 D for 1 and 2, respectively.
@article{Kellogg_2019,
doi = {10.1039/c8fd00201k},
url = {https://doi.org/10.1039%2Fc8fd00201k},
year = 2019,
publisher = {Royal Society of Chemistry ({RSC})},
volume = {216},
pages = {379--394},
author = {Michael Kellogg and Ali Akil and Daniel Sylvinson Muthiah Ravinson and Laura Estergreen and Stephen E. Bradforth and Mark E. Thompson},
title = {Symmetry breaking charge transfer as a means to study electron transfer with no driving force},
journal = {Faraday Discussions},
abstract = {Symmetry breaking charge transfer (SBCT) is a process where a symmetrically disposed pair of identical chromophores forms a charge transfer excited state with the hole and electron on different chromophores, i.e. chr–chr + hν → chr+–chr−. Herein we explore this process in two dipyrrin-based bichromophoric systems. One of these bisdipyrrins involved a pair of BODIPY chromophores linked by a single bond at their meso-positions (compound 1) and the other involved two dipyrrin ligands coordinated in a tetrahedral geometry at the Zn2+ ion (compound 2). Both compounds show rapid SBCT in polar solvents and only dipyrrin based emission in nonpolar solvents, the latter arising from a dipyrrin localized excited sate (LE). By “tuning” the solvent polarity the equilibrium between the LE and SBCT states can be shifted to favor either state. Ultrafast transient absorption spectroscopy (TA) was used to probe the kinetics of the charge transfer for 2 in solvents where the electron transfer is endergonic, exergonic and has a ΔG close to zero. Our TA derived rates were used to predict fluorescence efficiencies in each of the different solvent systems and showed a good correspondence to measured values. Detailed density functional theory (DFT) and time dependent DFT were used to model the ground states as well as the LE and SBCT states of 1 and 2, in both polar and nonpolar media. The ground and LE excited states show small dipole moments, while the SBCT states show dipole moments of 16.4 and 20.3 D for 1 and 2, respectively.},
bibbase_note = {<img src="https://pubs.rsc.org/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=C8FD00201K&imageInfo.ImageIdentifier.Year=2019">}
}Downloads: 0