Nature, 595(7869):673–676, Springer Science and Business Media LLC, 2021. 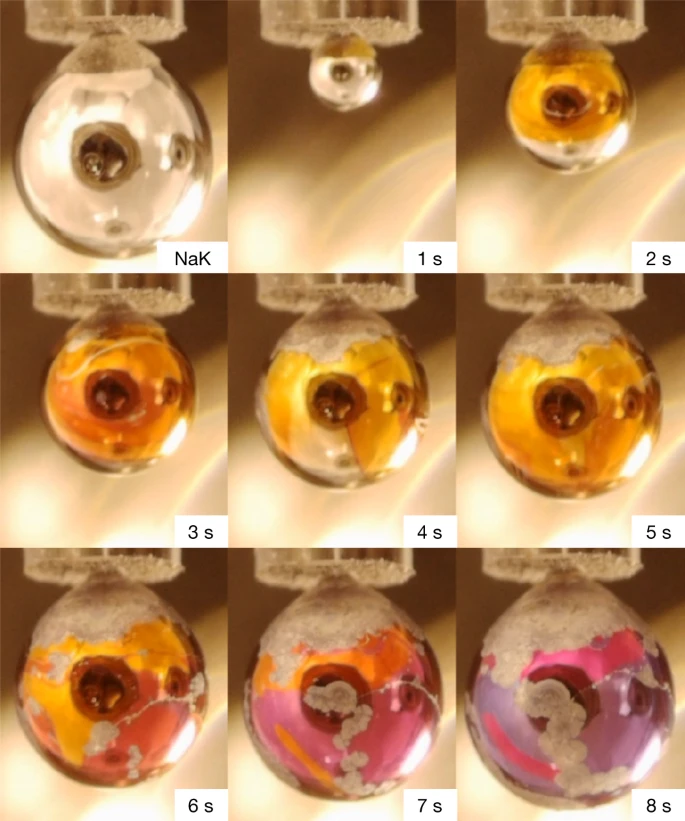
![link Spectroscopic evidence for a gold-coloured metallic water solution [link]](https://bibbase.org/img/filetypes/link.svg) Paper doi abstract bibtex 3 downloads
Paper doi abstract bibtex 3 downloads

![link Spectroscopic evidence for a gold-coloured metallic water solution [link]](https://bibbase.org/img/filetypes/link.svg) Paper doi abstract bibtex 3 downloads
Paper doi abstract bibtex 3 downloads Insulating materials can in principle be made metallic by applying pressure. In the case of pure water, this is estimated1 to require a pressure of 48 megabar, which is beyond current experimental capabilities and may only exist in the interior of large planets or stars2,3,4. Indeed, recent estimates and experiments indicate that water at pressures accessible in the laboratory will at best be superionic with high protonic conductivity5, but not metallic with conductive electrons1. Here we show that a metallic water solution can be prepared by massive doping with electrons upon reacting water with alkali metals. Although analogous metallic solutions of liquid ammonia with high concentrations of solvated electrons have long been known and characterized6,7,8,9, the explosive interaction between alkali metals and water10,11 has so far only permitted the preparation of aqueous solutions with low, submetallic electron concentrations12,13,14. We found that the explosive behaviour of the water–alkali metal reaction can be suppressed by adsorbing water vapour at a low pressure of about 10−4 millibar onto liquid sodium–potassium alloy drops ejected into a vacuum chamber. This set-up leads to the formation of a transient gold-coloured layer of a metallic water solution covering the metal alloy drops. The metallic character of this layer, doped with around 5 × 1021 electrons per cubic centimetre, is confirmed using optical reflection and synchrotron X-ray photoelectron spectroscopies.
@article{Mason_2021,
doi = {10.1038/s41586-021-03646-5},
url = {https://doi.org/10.1038%2Fs41586-021-03646-5},
year = 2021,
publisher = {Springer Science and Business Media {LLC}},
volume = {595},
number = {7869},
pages = {673--676},
author = {Philip E. Mason and H. Christian Schewe and Tillmann Buttersack and Vojtech Kostal and Marco Vitek and Ryan S. McMullen and Hebatallah Ali and Florian Trinter and Chin Lee and Daniel M. Neumark and Stephan Thürmer and Robert Seidel and Bernd Winter and Stephen E. Bradforth and Pavel Jungwirth},
title = {Spectroscopic evidence for a gold-coloured metallic water solution},
journal = {Nature},
abstract = {Insulating materials can in principle be made metallic by applying pressure. In the case of pure water, this is estimated1 to require a pressure of 48 megabar, which is beyond current experimental capabilities and may only exist in the interior of large planets or stars2,3,4. Indeed, recent estimates and experiments indicate that water at pressures accessible in the laboratory will at best be superionic with high protonic conductivity5, but not metallic with conductive electrons1. Here we show that a metallic water solution can be prepared by massive doping with electrons upon reacting water with alkali metals. Although analogous metallic solutions of liquid ammonia with high concentrations of solvated electrons have long been known and characterized6,7,8,9, the explosive interaction between alkali metals and water10,11 has so far only permitted the preparation of aqueous solutions with low, submetallic electron concentrations12,13,14. We found that the explosive behaviour of the water–alkali metal reaction can be suppressed by adsorbing water vapour at a low pressure of about 10−4 millibar onto liquid sodium–potassium alloy drops ejected into a vacuum chamber. This set-up leads to the formation of a transient gold-coloured layer of a metallic water solution covering the metal alloy drops. The metallic character of this layer, doped with around 5 × 1021 electrons per cubic centimetre, is confirmed using optical reflection and synchrotron X-ray photoelectron spectroscopies.},
bibbase_note = {<img src="https://media.springernature.com/lw685/springer-static/image/art%3A10.1038%2Fs41586-021-03646-5/MediaObjects/41586_2021_3646_Fig1_HTML.png?as=webp">}
}Downloads: 3